Registration of medicines
State registration of medicines is a prerequisite for the introduction of new products on the pharmaceutical market of the Republic of Azerbaijan.
PHARMEX offers you professional services for registration of medicines in the Azerbaijani Republic. State registration, re-registration, and changes in the registration dossier of the medicines is performed by the Analytical Examination Center under the Ministry of Health of the AR.
Medicine categories SUBJECT to state registration:
- original medicines
- generic medicines
- effective combinations of medicines that have been previously registered
- already registered medicines that have changed the pharmaceutical form and dosage
Medicine categories NOT SUBJECT to state registration:
- medicines prepared by pharmacies according to the appropriate recipe and with the fulfillment of all the requirements of medical organizations
- medicines purchased outside the territory of the Republic of Azerbaijan and intended for personal use
Registration Scheme of Medicines in Azerbaijan
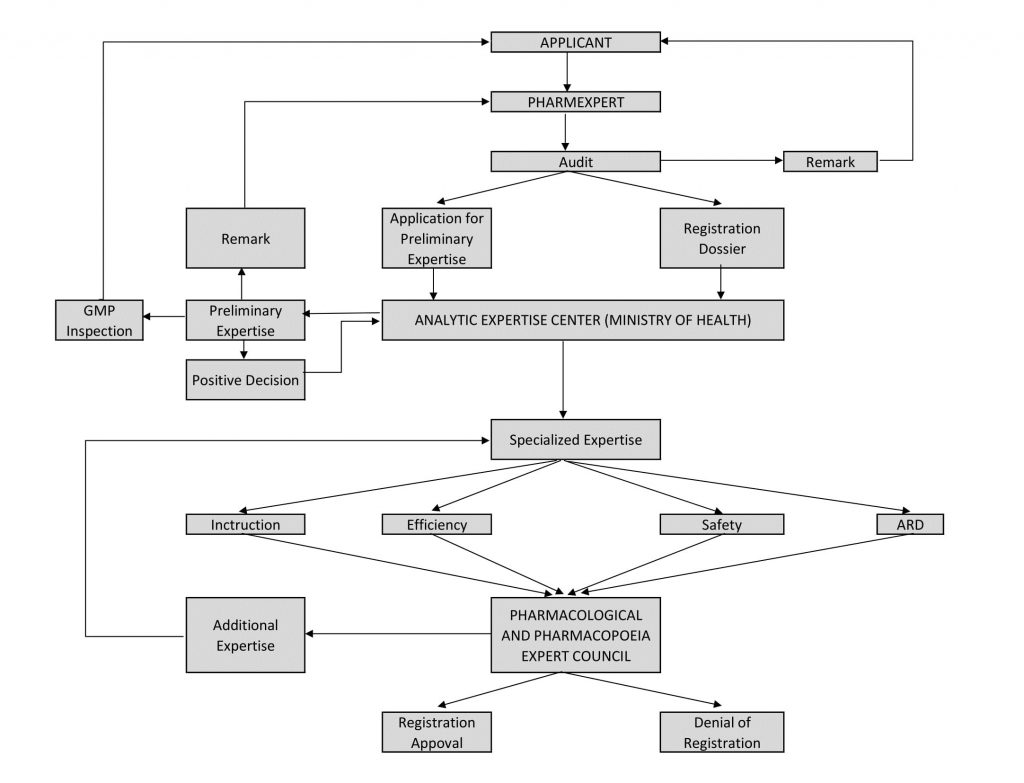
Upon completion of the registration process, a registration certificate is issued for the medicine and the registered medicine are then processed in the state register. After that, it is possible to sell the medicine in the Republic.
By turning to our specialists, you will receive qualified advice and assistance in the process of paperwork and passing the state registration procedure. We work right up to the moment of obtaining a registration certificate for medicines of a wide range of use and origin, as well as innovative and replicated drugs.
Registration deadlines of Medicines
In accordance with Decision No. 502 of Dec 25, 2020 with the Cabinet of Ministers of the AR
| Description of Procedures | Time Range |
| Concluding an Agreement (Preliminary Expertise) | 5 days |
| Payment of Duty (Preliminary Expertise) | 15 bank working days |
| Preliminary Expertise | 15 days |
| Responds of Applican to Remarks of Preliminary Expertise (if available) | 90 days |
| Concluding an Agreement (Specialized Expertise) | 5 days |
| Payment of Duty (Specialized Expertise) | 60 days |
| Specialized Expertise (Registration) | 210 days |
| Specialized Expertise (On Renew) | 90 days |
| Specialized Expertise (On Registration of Amendments) | 90 days |
| Responds of Applican to Remarks of Specialized Expertise (if available) | 90 days |
| Decision of Expert Board | 10 days |
| Additional Specialized Expertise (if necessary) | 30 days |
| Responds of Applican to Remarks of Additional Specialized Expertise (if available) | 90 days |
| Final Decision of Expert Board | 15 days |
| Issuing of Certificate | 15 days |
Registration process may delay due to objective reasons. The above timing does not include the inspection of the production site.
Our specialists are engaged not only in the standard registration procedure, but also provide services for making changes and confirming the state registration of medicines. Our employees work according to a well-coordinated scheme of actions for solving typical tasks and problems that arise at all stages of paperwork and registration.
Changes that require expertise:
• if necessary, change the instructions for use of the medicine
• replacing of excipients to the composition of the medicines
• when changes are made to data on the manufacturer of a medicine;
• when changing indicators that characterize the quality of the drug, as well as the replacement of methods for controlling the quality of the drug;
• at change of the expiration date established for the registered medicine.
All of the above changes must be introduced within a maximum of 3 months from the date of the decision being made about the change by manufacturers.
Refusal of Registration
The Ministry of Health refuses state registration of medicines in the following cases:
• If the information provided in the submitted documents is not true;
• The medicine contains a prohibited substance in the territory of the Republic of Azerbaijan;
• If the quantitative and qualitative indicators specified in the submitted documents do not correspond to reality;
• If therapeutic efficacy is not proven;
• Clinical trials and other studies evaluating the safety, efficacy, and quality of a drug are unsatisfactory.
• If serious side effects are detected during the registration process;
• Negative opinion on the results of the manufacturer’s verification;
• Negative opinion on the results of specialized expertise and (or) on the results of additional specialized expertise conducted by the Expert Council on Pharmacology and Pharmacopoeia of the Ministry of Health of the Azerbaijan Republic.
When making a decision to refuse the state registration of a medicine, the applicant is provided with a written reasoned response.
Terms of re-registration
According to the law, for state re-registration of the drug, the applicant should contact the Agency at least 210 (two hundred and ten) calendar days before the expiry of the previous state registration period.